Stablepharma Ltd’s “Fridge-Free” Vaccine Trials in the UK



Here’s a detailed breakdown of the development and significance of fridge-free vaccines in the UK.
What is happening
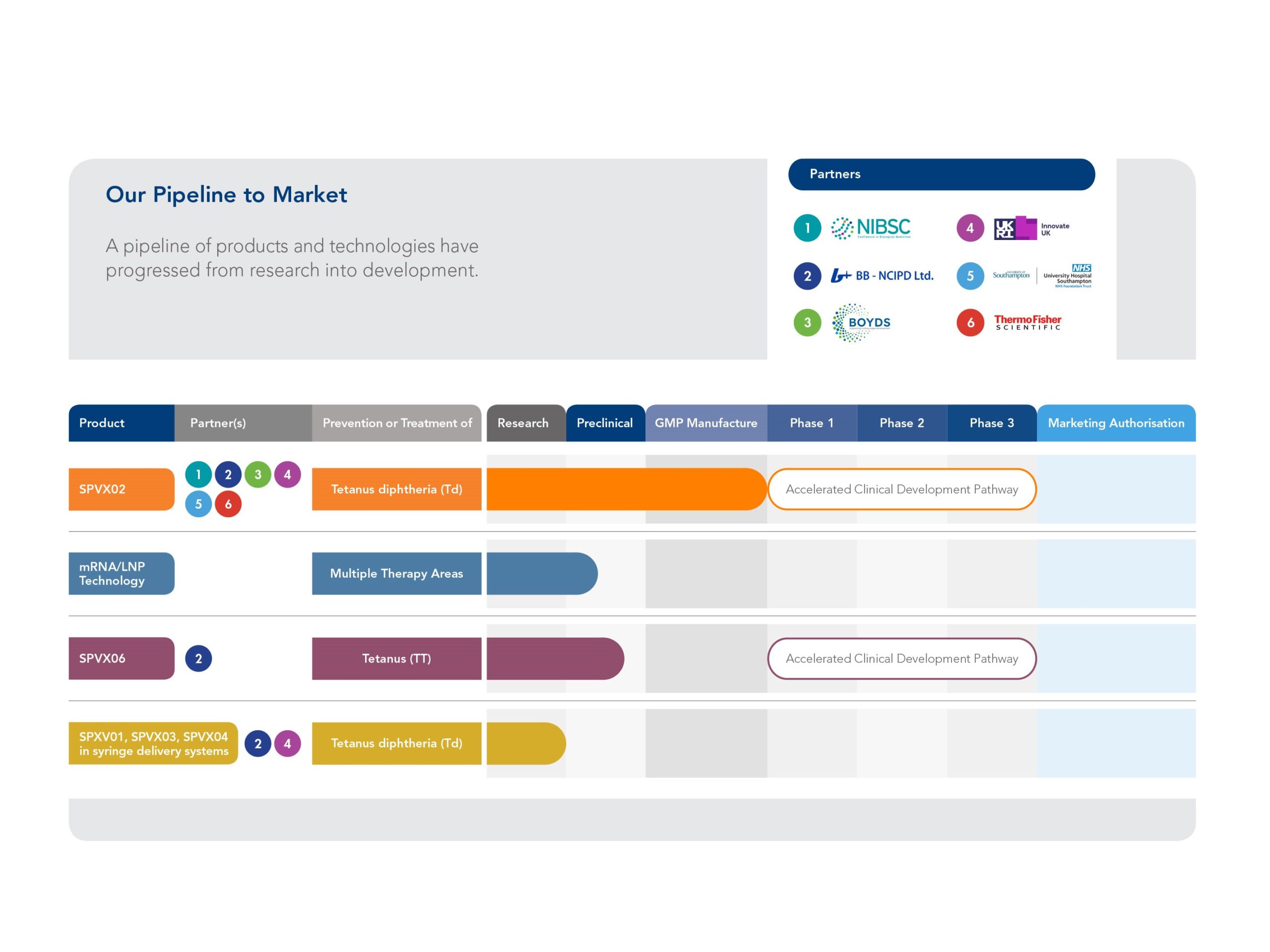
- Stablepharma has developed a lead vaccine candidate, SPVX02, a tetanus-&-diphtheria (Td) booster, reformulated using its proprietary platform (StablevaX™) into a thermostable vaccine that does not require continuous refrigeration. (Stablepharma)
- The UK regulatory body Medicines and Healthcare products Regulatory Agency (MHRA) granted approval for a Phase 1 first-in-human clinical trial in the UK. (bioindustry.org)
- The trial is run at the NIHR Southampton Clinical Research Facility (University Hospital Southampton) under lead investigator Saul Faust and Stablepharma’s CDO Karen O’Hanlon. (ITVX)
- The first participant was dosed in April 2025 (for example, 15 April 2025) and the trial is expected to complete in Q3 2025, with results by end of year. (BioSpace)
- Prior to this, Stablepharma had shown in stability studies that SPVX02 remained fully potent after exposure to extreme temperature fluctuations (-20 °C up to +40 °C) and claimed a shelf-life at room temperature for the clinical batch. (BioSpace)
- They claim their technology could be applied to up to 60 other vaccines which currently rely on a cold-chain. (The Standard)
Why it matters
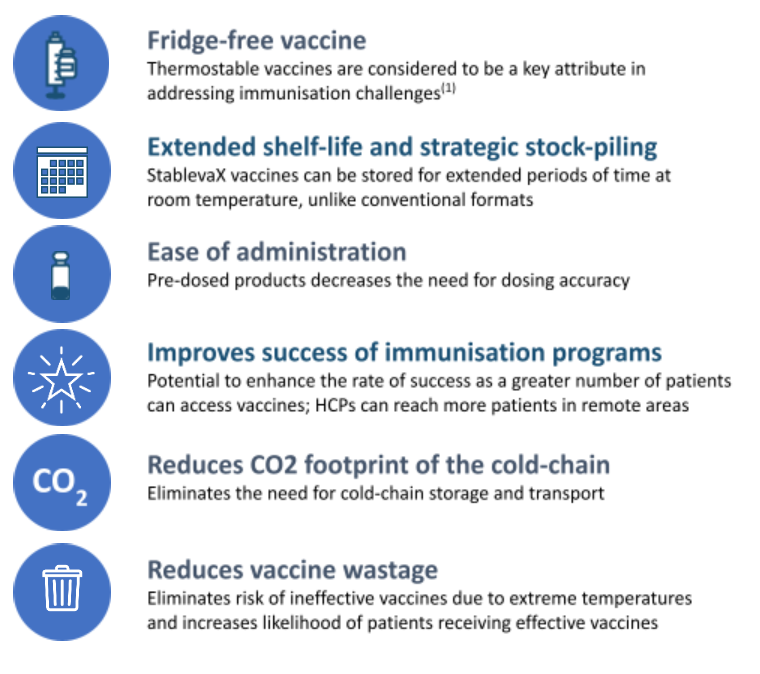
- Cold-chain reduction: Vaccines typically require storage at 2–8 °C or even lower in some cases. The requirement for refrigeration/frozen transport makes distribution costly, energy intensive, and risky (storage failures → wastage). The WHO estimates up to ~50% of vaccines are wasted annually (globally) partly due to cold-chain failures. (ITVX)
- Access in low-resource settings: A vaccine that can be stored at ambient/room temperature dramatically improves reach in remote, rural or low-income regions where constant refrigeration is difficult. That can enhance immunisation coverage, equity and global health impact.
- Cost savings & carbon footprint: Reducing reliance on refrigeration and cold transport reduces logistics cost, energy use and CO₂ emissions—an added sustainability benefit. (BioSpace)
- UK biotechnology leadership: The trial being the “world-first” human trial of a fridge-free vaccine positions the UK as a leader in this niche of vaccine innovation. Public funding and regulatory support underline this. (The Standard)
Key details and metrics
| Parameter | Value / detail |
|---|---|
| Technology platform | StablevaX™ – reformulates existing/new vaccines into thermostable format. (Stablepharma) |
| Vaccine candidate | SPVX02 – Td (tetanus & diphtheria) adult booster. (Stablepharma) |
| Trial phase | Phase 1, first‐in‐human, UK. (bioindustry.org) |
| Location | NIHR Southampton Clinical Research Facility, University Hospital Southampton. (ITVX) |
| Stability claim | Remains stable over multiple cycles between -20°C and +40°C. Room temperature shelf-life for clinical batch: ~18 months (pre-commercial claim). (Healthcare Today) |
| Future pipeline | Up to ~60 approved or under-development vaccines could be eligible for reformulation via the platform. (The Standard) |
| Potential global use date | Company hope for global use by 2027 (if trial and further development succeed). (The Standard) |
What to watch / next steps
- Safety & immunogenicity outcomes: The Phase 1 trial will primarily test safety and tolerability (and some immunogenicity) in a small adult cohort. The results—when published—will be critical. Until those data are peer-reviewed, the claims remain promising but unproven.
- Scale-up & manufacturing: Even if thermostability works, manufacturing at GMP scale, regulatory approvals, and distribution logistics still need to be addressed. The company claims scalable GMP manufacturing. (BioSpace)
- Regulatory & global policy acceptance: Will regulatory agencies globally accept thermostable vaccines based on new formulation platforms? Will WHO prequalification recognise them? These are important for global impact.
- Cost vs benefit: Although thermostable vaccines reduce cold‐chain cost, the reformulation cost and licensing must be economically viable for manufacturers and health systems.
- Application to other vaccines: While the Td vaccine is a proof-of-concept, applying to high-profile global vaccine targets (e.g., childhood immunisations, mRNA vaccines, COVID-19 boosters) is a larger challenge.
- Market dynamics & access: A major benefit is access in low-resource settings—but models for equitable distribution, pricing and manufacturing in such settings will matter.
- Data transparency & real-world testing: The durability claims (-20°C to +40°C cycles, 18-month shelf life) are strong. Independent verification and public data will boost confidence.
Comments & reflections
- Innovative step: This is one of those “incremental but transformative” innovations: not a new antigen, but a new formulation/ logistics twist that could unlock large value (“what if vaccines didn’t need a fridge?”).
- Global health game-changer potential: From a global perspective, removing cold-chain burden is massive. Many low-income countries struggle with last-mile refrigeration; a fridge-free vaccine could significantly increase coverage, reduce wastage and cost.
- UK biotech ecosystem strength: The fact that a UK company, supported by UK public funding (Innovate UK, NIHR) is leading this trial is positive for the UK’s life-sciences ecosystem. It shows “beyond the big vaccines” innovation is alive.
- But not guaranteed success: The history of vaccine innovation cautions that early promise doesn’t always translate into widely used products. Stability in the lab and batches doesn’t always map to real‐world supply chain, manufacture, regulation, and distribution complexity.
- Broader implications for vaccine logistics: If successful, this could prompt a shift in how vaccines are designed and deployed—thinking about thermostability from the start, not just antigen efficacy. That might influence future vaccine development strategies (including for pandemics).
- Commercial & public-health intersection: The reformulation could open new commercial opportunities (licensing, platform technology) but also raises questions of access and pricing—especially for global health. Balancing innovation with equitable access will be important.
- Policy & sustainability benefits: Reducing cold‐chain also means less energy use, less waste, fewer spoiled doses—so there is a sustainability and cost-saving dimension that complements health benefits.
- Timing is good: With vaccine equity, pandemic preparedness, supply-chain resilience high on global radar, the timing for such innovation is opportune. If the trial succeeds, it may accelerate interest and investment in similar formulation/thermostability technologies.
Final summary
The UK’s launch of a “fridge-free” vaccine trial (SPVX02 by Stablepharma) is a noteworthy and potentially game-changing development in vaccine logistics and global health. While still early—pending Phase 1 results—it addresses a key bottleneck (cold-chain dependency) and holds promise for broader application. For innovators, manufacturers, global health policymakers and investors alike, it’s a technology to monitor closely.
Here are two detailed case studies of fridge-free vaccine trials in the UK and a commentary section on what they imply for the broader vaccine/health-innovation ecosystem.
Case Study 1: Stablepharma Ltd – SPVX02 (Tetanus & Diphtheria)


Background & Technology
- Stablepharma, a UK-based biotech, has developed a technology platform called StablevaX™ which reformulates vaccines/biologics into thermostable formats that no longer require the conventional “cold chain” (refrigeration at 2-8 °C or freezing). (bioindustry.org)
- According to publicly-available data, their lead candidate SPVX02 (a tetanus & diphtheria (Td) adult booster) remains stable at room temperature (up to ~30 °C) with an 18-month shelf life for the clinical batch. It also withstands extreme temperature fluctuations (−20 °C to +40 °C) in stability testing. (Stablepharma)
Trial Details
- The UK regulator Medicines and Healthcare products Regulatory Agency (MHRA) approved the Phase 1 first-in-human trial for SPVX02. (bioindustry.org)
- The trial is being conducted at the NIHR Southampton Clinical Research Facility at University Hospital Southampton under lead investigator Saul Faust in partnership with Dr Karen O’Hanlon (Stablepharma). (uhs.nhs.uk)
- First participant was dosed on 15 April 2025. The Phase 1 trial is expected to complete by Q3 2025, with initial data by end of 2025. (Stablepharma)
Why it matters
- The “cold chain” (continuous refrigeration/tracking) is a major global barrier: the World Health Organization (WHO) estimates up to 50% of vaccines are wasted annually because of storage/distribution issues. (The Independent)
- If SPVX02 and its underlying platform perform as claimed, you could see major improvements in vaccine access, equity, cost-reduction, and logistics especially in low-resource or remote settings.
- The UK is positioning itself as a leader in this area: the government is publicly supporting the trial. (The Independent)
Key metrics & claims
- Temperature stability: fully potent after three cycles of −20 °C to +40 °C. (clinicaltrialsarena.com)
- Shelf life: 18 months at up to ~30 °C for the clinical batch. (Stablepharma)
- Pipeline potential: Up to ~60 other existing temperature-sensitive vaccines could be reformulated via the platform. (clinicaltrialsarena.com)
Case Study 2: Regional / Infrastructure Lens – UK Government-backed Programme & Cold Chain Disruption


Context
- The cold-chain requirement (2-8 °C, sometimes −20 °C or lower) is costly: transport, storage, monitoring, waste due to breaks in chain. The UK’s funding programmes (via Innovate UK, NIHR etc) have supported innovation to overcome these logistic bottlenecks. For example, Stablepharma’s trial is part-funded via UK government schemes. (Stablepharma)
- The UK’s NIHR Southampton facility is hosting the “world-first” human trial of a fridge-free vaccine (SPVX02) and emphasises the potential global impact of such tech. (CRF)
Why this is a system-level case
- It shows how innovation is not only about new antigens/immunology, but also formulation & logistics.
- It underscores the UK ecosystem: biotech firms + national research institutes + government backing + NHS infrastructure collaborating.
- If successful, this kind of innovation could ripple across many vaccines (not just new ones) by making existing ones easier to store/distribute.
Commentary – What the Rise of Fridge-Free Vaccines Means
Positive implications
- Increased access & equity: Reducing reliance on the cold chain means vaccines could reach more remote, resource-constrained areas; distribution becomes simpler and less costly.
- Cost & waste reduction: Less need for refrigeration, lower energy/transport logistics, fewer spoiled vaccines. That saves money and improves public-health return on investment.
- UK innovation leadership: The UK is showing early institutional leadership in this niche—potential export strength, biotech growth, global health credentials.
- Systemic innovation: Not just new vaccines, but existing ones reformulated—this multiplies impact. The case of up to 60 candidate vaccines for reformulation shows this potential.
Key caveats / challenges
- Proof-of-concept is early: Phase 1 trials test safety/immunogenicity primarily; broader efficacy, commercial scale-up, regulatory approvals remain to happen. Until then, claims remain promising rather than proven.
- Manufacturing & supply-chain still matter: Even if thermostability is achieved, large-scale GMP manufacturing, regulatory acceptance, global distribution (licensing, local regulation) must be addressed.
- Cost of reformulation/licensing: Converting an existing vaccine into a new format may incur R&D/manufacturing cost. The economics must make sense for pharma manufacturers and global health purchasers.
- Regulatory & acceptance issues: Regulatory bodies in different countries, WHO pre-qualification, national immunisation programmes will need evidence of performance, safety, cost-benefit.
- Not all vaccines may be amenable: Some vaccine types or biologics may be harder to thermostabilise; cold chain may still be required for many. So it’s a significant improvement, but not a universal panacea.
Strategic implications
- For biotech/start-ups: Focus on formulation innovation (thermostability) as a high-impact differentiator. Partner with established vaccine manufacturers, global health agencies, regulators early.
- For global health policy: Investment in these kinds of innovations helps reach underserved populations and achieves immunisation goals (e.g., WHO’s Universal Health Coverage). Governments and NGOs should monitor and invest in these pipeline technologies.
- For manufacturing/investment: Investors and manufacturers should view this as an emerging category: vaccines that avoid cold chain present a new market dynamic (e.g., in low-resource countries, or for stockpiling/emergency use).
- For national biotech strategy: Countries that develop and export this capability may gain a competitive advantage—not only scientifically but economically (manufacture, export, licensing). The UK is aiming for that position.
Final Take-away
The trial of the fridge-free vaccine (SPVX02) in the UK is not just an incremental advancement, but has the potential to reshape vaccine delivery logistics globally. It addresses a major barrier—the cold chain—one that contributes to high waste, high cost, and inequitable access. The UK case shows how formulation innovation + national support + clinical infrastructure converge.
However: the innovation still has to prove itself (safety, efficacy, scalability, cost-viability, global acceptance). If it succeeds, it could open a new chapter in vaccines — simpler distribution, broader access, lower waste, and maybe lower cost.

